| |
| |
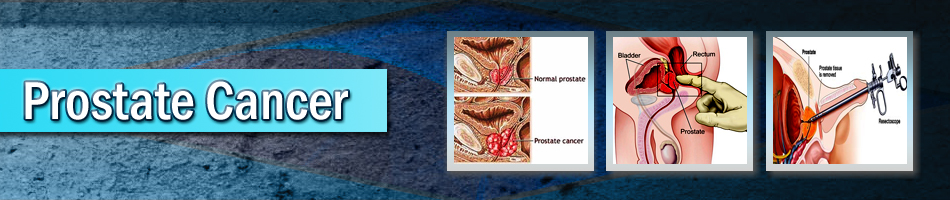
| Prostate Cancer |
|
|
Prostate Cancer is the second-leading cause of cancer deaths among men in the United States and a significant health-care problem due to its high incidence. It is estimated that in the United States (U.S.), there will be more than 238,590 new cases and 29, 720 deaths from prostate cancer in 2013. The natural history and progression of this disease is not clearly and consistently understood. An analysis of autopsy studies has shown that approximately one in three men over the age of 50 years had histologic evidence of prostate cancer, with up to 80% of these tumors being limited in size and grade and, therefore, clinically insignificant. However, a recent study of incidental prostate cancer diagnosed in organ donors found prostate cancer in 1 in 3 men age 60-69, and this increased to 46% in men over age 70.
The number of men diagnosed with prostate cancer remains high. However, 5-year relative survival rates have increased dramatically over the years. There also has been at least a 25% reduction in the age-specific prostate cancer mortality rate since the beginning of the PSA era. It is estimated that 99 % of men diagnosed with localized or regional prostate cancer survive at least five years, while only 33% of those with metastases at diagnosis survive 5 years.
For African American men, however the statistics are more dramatic. African American men when diagnosed are more likely to be diagnosed at a later stage and 2.4 times more likely than white men to die of prostate cancer. In addition, if you have a family history the risk is even greater.
Thus, it is still important for men to know the facts about prostate cancer and have an informed discussion with their doctors about prostate cancer testing. For information on prostate cancer please visit the page for our Know Your Stats About Prostate Cancer® campaign, in partnership with the National Football league.
|
| |
|
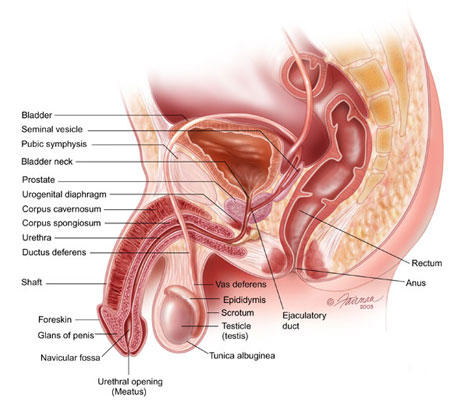
What is the prostate? |
|
|
|
The prostate is part of the male reproductive system and is a small, walnut-sized gland that sits below the bladder and in front of the rectum. The prostate gland secretes a fluid that makes up part of the semen.
Men should discuss with their physicians about their need to have a prostate cancer screening; which helps maintain proper prostate health. |
| |
|
| What are the causes and risks associated with prostate cancer? |
|
|
What exactly causes prostate cancer is still unknown; however, the scientific community is conducting research with the hope of finding the answer soon. The current theory is there are many factors that can increase a man’s risk for prostate cancer.
The following are some factors:
• Age
• Smoking
• World Region Location
• Ethnicity – being African American doubles your risk
• Family History
• Dietary
• Vitamins
The disease predominately affects older men and is rarely found in men younger than 40. Approximately 1 in 35 men will die of prostate cancer with African American’s twice as likely as Caucasian men to die of the disease. According to the American Cancer Society prostate cancer accounts for about 10 percent of cancer-related deaths in men between the ages of 60 and 79 and nearly 25 percent in those over the age of 80.
As men age, their risk of developing prostate cancer increases. If you are a heavy smoker, studies have shown that your risk of prostate cancer may double. The good news is the risks decreases to roughly that of a non-smoker of the same age within 10 years of quitting.
Worldwide, prostate cancer ranks third in cancer incidence and sixth in cancer mortality among men. There is, however, a notable variability in incidence and mortality among world regions. The incidence is low (but rapidly increasing in recent years) in Japan and other Asian countries and intermediate in regions of Central America and Western Africa. The incidence is higher in North America and Northern Europe. However, the higher rates in North America and Northern Europe can be due to the different screening practices, genetic predisposition, diet and environmental factors.
African-Americans are in the highest risk group, with an incidence of more than 200 cases per 100,000 black men. While the incidence in Caucasian and Asian men is slightly more than half that of blacks, African-American men tend to present with more advanced disease and have poorer overall prognosis than Caucasian or Asian men.
Men with a family history of prostate cancer are at an increased risk of developing the disease, the more first-degree relatives the higher a man’s risk of developing the disease. In addition, the age of onset the first-degree relatives was diagnosed can increase a man’s risk of developing the disease. Men with a family history of disease are 2 to 11 times more likely to develop prostate cancer then men without a family history of prostate cancer.
There is also considerable evidence showing a Western lifestyle is associated with increased prostate cancer risk and increased death from prostate cancer. However, which specific lifestyle factor is unknown. Engaging in excessive calorie, dietary fat and refined sugar intake with reduced fruit and vegetable and exercise activity is shown to increase the risk of prostate cancer, though the relationship is not entirely clear. However, of those the most commonly cited dietary risk factor is a high intake of dietary fat, though that relationship is still unclear. Being obese is associated also with an increased risk for death from prostate cancer. Thus, the simplest advice for avoiding death from prostate cancer is to prevent obesity and if you are obese, to lose weight and keep it off.
There is a limited amount of evidence to suggest that the worldwide difference in prostate cancer incidence may be associated with dietary intake of soy proteins in other parts of the world. In Asian countries such as Japan and the Republic of Korea where prostate cancer incidence and mortality are just a fraction of that in North America, soy consumption in the form of tofu, soy milk and miso is up to 90 times higher than that consumed in the United States. In a study of more than 40 nations, researchers found soy, on a per calorie basis, to be the most protective dietary factor. This protective role may be associated with two of soy's components, genistein and daidzein that may act as weak estrogens. Estrogens are female hormones that inhibit prostate cancer growth. Some experts have suggested that the worldwide differences in prostate cancer incidence may also be explained by the high intake of green tea by residents of Asia. However, determining which factors from a complex dietary mix cause prostate cancer is not easy and no clear answers have emerged.
The intake of other certain dietary factors such as lycopene and fish oil may also reduce the risk of developing prostate cancer. Cooked tomatoes are rich sources of lycopene. Lycopenes are antioxidants that may protect cells from becoming cancerous. Several studies have shown that the likelihood of developing prostate cancer is reduced by high intake of lycopene. Researchers found men ingesting two or more servings of tomato sauce per week had a 36 percent reduction in cancer risk compared to those who did not, however again, not all studies have supported this. Fish oils (omega-3 fatty acids) are thought to reduce heart disease due to reducing inflammation. Given the presumed importance of inflammation in causing prostate cancer, it stands to reason that fish oils may prevent prostate cancer. Indeed, some studies have suggested this, though others have failed to find any link with prostate cancer risk.
Although some studies in the past have suggested that men who had undergone a vasectomy were at an increased risk of developing prostate cancer, newer studies have indicated that vasectomy is not a risk factor for prostate cancer.
Attention has also focused on vitamin D's effect on the prostate. Epidemiologic evidence shows an inverse relationship between prostate cancer risk and ultraviolet radiation, the primary source for vitamin D production. This observation has led some to suggest that higher rates of prostate cancer in the elderly may be partly due to decreased sun exposure or a decline in the body's ability to make vitamin D with aging. However, several recent studies have found no correlation between vitamin D levels and prostate cancer risk and one even found that men with increased vitamin D had a higher risk of aggressive prostate cancer!
Finally, a word of caution is needed. Based upon very exciting data, the National Institute of Health embarked a large randomized trial of over 30,000 men to test whether vitamin E or selenium would prevent prostate cancer. Unfortunately, the trial was stopped early because there was no evidence either agent alone or in combination prevented prostate cancer. Moreover, there was a suggestion that men who took vitamin E had an increased risk of prostate cancer and men who took selenium had a slightly higher risk of diabetes! This highlights the point that there is no easy substitute for a healthy lifestyle involving eating a balanced diet, avoiding dietary excesses, eating plenty of fruits and vegetables, getting lots of exercise, and most importantly achieving and maintaining a normal body weight.
While there are no exact causes of prostate cancer most doctors agree, if you do things that are heart healthy, you will also keep your prostate healthy. Eating right, exercising, watching your weight and not smoking can improve your health and help them avoid this disease.
|
| |
|
| What are the symptoms of prostate cancer? |
|
|
In its early stages, prostate cancer often causes no symptoms. When symptoms do occur, they may include any of the following:
• dull pain in the lower pelvic area;
• frequent urination;
• problems with urination such as the inability, pain, burning, weakened urine flow;
• blood in the urine or semen;
• painful ejaculation;
• general pain in the lower back, hips or upper thighs;
• loss of appetite and/or weight;
• persistent bone pain
Fill out the AUA Symptom Score and share the results with your health care provider.
|
| |
|
| How is prostate cancer diagnosed? |
|
|
Currently, digital rectal examination (DRE) and prostate specific antigen (PSA) are used for prostate cancer detection.
DRE: The DRE is performed with the man either bending over, lying on his side or with his knees drawn up to his chest on the examining table. The physician inserts a gloved finger into the rectum and examines the prostate gland, noting any abnormalities in size, contour or consistency. DRE is inexpensive, easy to perform and allows the physician to note other abnormalities such as blood in the stool or rectal masses, which may allow for the early detection of rectal or colon cancer. Because the DRE by itself is not an effective way to detect early cancer, it should always be combined with a PSA test.
Prostate Specific Antigen Test (PSA): The PSA test is usually performed in addition to DRE. The test measures the level of PSA, a substance produced only by the prostate, in the bloodstream.
The blood test can be done in a clinical laboratory, hospital or physician's office and requires no special preparation on the part of the patient. Ideally, the test should be taken before a DRE is performed or any catheterization or instrumentation of the urinary tract. Furthermore, because ejaculation can transiently elevate the PSA level for 24 to 48 hours, the patient should abstain from sexual activity for two days prior to having a PSA test.
Very little PSA is detected from patients with a healthy prostate, but certain prostatic conditions can cause larger amounts of PSA to leak into the blood. One possible cause of a high PSA level is benign (non-cancerous) enlargement of the prostate, otherwise known as BPH. Inflammation of the prostate, called prostatitis is another common cause of PSA elevation, as is recent ejaculation. Prostate cancer is the most serious possible cause of an elevated PSA level. The frequency of PSA testing remains a matter of some debate.
The American Urological Association (AUA) believes that the decision to screen is one that a man should make with his doctor following a careful discussion of the benefits and risks of screening.
|
| |
|
| What happens if my PSA and/or DRE are abnormal? |
|
|
If you have an abnormal prostate cancer screening the only way to determine if you have, prostate cancer is through a biopsy. The decision to proceed with a prostate biopsy should be based primarily on PSA and DRE results. It should also take into account other factors including your family history of prostate cancer, race, any prior biopsy history and other significant health issues you may have. A prostate biopsy is best performed under transrectal ultrasound guidance using a spring-loaded biopsy device coupled to the transrectal probe.
Patients are positioned on their side for this procedure and are given an enema and an antibiotic. The lubricated ultrasound probe is inserted into the rectum. The physicians will first us the ultrasound to find the prostate gland particularly focusing on the size and shape and whether or not any other abnormalities. The most common abnormalities are shadows, which might signify the presence of prostate cancer. However, not all prostate cancers are visible. After the prostate gland has been anesthetized with an injection of a local anesthetic through a long fine needle that is passed through the probe, the physician performs the biopsy. Using the spring-loaded biopsy device attached to the ultrasound probe, the physician removes several pieces of the prostate gland. Generally, 10 to 12 pieces or cores are removed (or more, depending upon the size of the prostate gland and the prior PSA and biopsy history of the patient). Each core of prostate tissue is approximately 3/4 inch in length and 1/16 inch in width. The entire procedure takes 20 to 30 minutes. The removed tissue is taken and will be examined by a pathologist (a physician who specializes in examining human tissue to determine whether it is normal or diseased). The pathologist will be able to confirm if cancer is present in the biopsy tissue. If cancer is present, the pathologist will also be able to grade the tumor. The grade indicates the tumor's degree of aggressiveness—how quickly it is likely to grow and spread.
There transrectal ultrasound guided prostate biopsy is usually well tolerated. Using local anesthetics helps to minimize the discomfort associated with the biopsy. There are some side effects that may result from the biopsy such as, blood in the ejaculate (hematospermia) and/or blood in the urine (hematuria) however, it should clear up within a few days for the urine and a few weeks for the semen. High fever is rare, occurring in only 1 to 2 percent of patients. The antibiotic is continued for at least 48 hours after the biopsy procedure |
| |
|
| How does the Gleason Score work? |
|
|
The Gleason grading system is the most widely used system. In this system, because often several different tumor patterns are seen, the most common tumor pattern is assigned a score from 1 to 5 and the second most common pattern is similarly assigned a score, using the same scale. The two scores are added together to give a Gleason sum ranging between 2 and 10. Scores of 2 to 6 designate mildly aggressive prostate cancer, 7 moderately aggressive and scores of 8 to 10 are highly aggressive cancers.
|
| |
|
| How is prostate cancer staged? |
|
|
The T stage is divided into the following categories:
T1: Doctor is unable to feel the tumor
T1a: Cancer is found incidentally during a transurethral resection (TURP) for benign prostatic enlargement. Cancer is present in less than 5% of the tissue removed and is low grade (Gleason < 6)
T1b: Cancer is found after TURP but is present in more than 5% of the tissue removed or is of a higher grade (Gleason > 6)
T1c: Cancer is found by needle biopsy that was done because of an elevated PSA
T2: Doctor can feel the tumor when a digital rectal exam (DRE) is performed but the tumorstill appears to be confined to the prostate
T2a: Cancer is found in one half or less of only one side (left or right) of the prostate
T2b: Cancer is found in more than half of only one side (left or right) of the prostate
T2c: Cancer is found in both sides of the prostate
T3: Cancer has begun to spread outside the prostate and may involve the seminal vesicles
T3a: Cancer extends outside the prostate but not to the seminal vesicles
T3b: Cancer has spread to the seminal vesicles
T4: Cancer has spread to adjacent organs, such as the urethral sphincter, rectum, bladder, and/or wall of the pelvis
Imaging tests, such as radionuclide bone scan, CT scan, MRI, and MR spectroscopy may help assess whether the cancer is still confined to the prostate or spread elsewhere. To determine if the cancer has spread to the lymph nodes or bones, the physician may order a CT or MRI scan of the pelvis. Sometimes follow-up images are needed to evaluate abnormalities found on the bone scan. These tests are not recommended for men with a Gleason grade lower than 7 and a PSA level lower than 10 ng/ml as they rarely show disease.
|
| |
|
| Are there tests to determine if my cancer has metastasized? |
|
|
To determine if your cancer has spread to other parts of your body your doctor may recommend the following:
• A pelvic CT scan
• MRI scan
• Bone scan
Not all men with prostate cancer need to undergo imaging tests as the risk of spread to other organs can be estimated by PSA levels and cancer grade. It is also standard to omit the bone scan in patients for the following reasons:
• Newly diagnosed, untreated prostate cancer,
• Patients who have no symptoms from their cancer,
• Gleason score of less than 7 and have serum PSA concentrations less than 10 ng/ml
• PSA concentrations less than 15 ng/ml (unless the Gleason score is 7 or higher)
A pelvic CT scan or MRI may not be necessary in men with lower grade cancers, cancers still confined to the prostate, and serum PSA values less than 10 ng/ml.
|
| |
|
| Can prostate cancer be prevented? |
|
|
There is still a lot of controversy regarding the prevention of prostate cancer. Some physicians believe anti-androgen drugs, such as finasteride and dutasteride, can prevent prostate cancer. However, others are skeptical, and believe anti-androgens only slow the progression of well-differentiated tumors while allowing higher-grade elements to emerge as the dominant elements in the tumor. In randomized trials, men taking these drugs were less likely to be diagnosed with prostate cancer. However, whether the drug’s use will affect the cancers aggressiveness and translate into a lower death risk is still unknown. Some physicians believe that general health measures might reduce the risk of prostate cancer, such as eating and maintaining a normal body weight, a healthy diet, being physically active and visiting the doctor on a regular basis. However, the best practice to prevent prostate cancer is to live and practice a healthy lifestyle.
|
| |
|
| What are the current treatment options for localized prostate cancer? |
|
|
Because not all prostate cancer is the same and not all are life threatening, it is important to understand the treatment options you choose should be very specific to your personal health history. It is an individual decision that patients should make together with their doctor and their family. No one treatment is perfect for every man.
Several factors come into play when selecting the best treatment for an individual and they include:
• Tumor stage (extent of local spread) and grade (aggressiveness),
• PSA level (higher levels indicating a greater risk of cancer recurrence after treatment), and extent of disease (number of biopsy cores with cancer), as well as
• competing medical co-morbidities (other diseases that can affect life expectancy) and
• Age at diagnosis (as most prostate cancers take many years to become evident and cause morbidity, the same tumor in an older man may pose a lower risk of causing problems in his lifetime); all play a role in the decision regarding the choice of therapeutic intervention
It is important that you speak to your doctor about which treatment is right for you.
Below is a list of the common medical treatments for localized prostate cancer:
• Active Surveillance
• Surgery
• Radiotherapy
• Cryotherapy |
| |
|
Active Surveillance
What is Active Surveillance? |
|
|
Prostate cancer is often a slow progressive disease, and many men with prostate cancer will die from causes other than prostate cancer. Your physician may recommend the active surveillance treatment option if you have been diagnosed with a very early stage of prostate cancer.
Active surveillance is a type of close follow-up for men with prostate cancer. This follow-up usually involves regular prostate-specific antigen (PSA) tests, digital rectal examinations (DREs) and possible prostate biopsies. If these periodic tests show that your cancer is progressing, you and your doctor may begin to discuss other forms of treatment.
The goal of active surveillance is to allow men to maintain their quality of life when the prostate cancer is slow growing or inactive, while allowing them to elect active treatment when the disease becomes more aggressive or begins to grow. |
| |
|
| Who is a good candidate for Active Surveillance? |
|
|
Active surveillance may be a suitable option for men who are not experiencing symptoms, or when the cancer is not expected to grow at an aggressive rate. Active surveillance allows men to maintain their quality of life, thus for those whom avoidance of sexual, urinary, and/or bowel complications are a primary consideration, active surveillance may be considered. Active surveillance can also be considered for men who are older or have other serious health conditions, which can make the cancer more difficult to treat immediately.
What are the advantages and disadvantages of Active Surveillance?
There are two advantages for utilizing this treatment option – low cost and no immediate complications.
The risk of active surveillance is that the cancer could grow and spread to other parts of the body between follow-ups, making it more difficult to treat. Not all prostate cancers require active treatment, and not all prostate cancers are life threatening. The decision to implement active treatment is one a man should discuss in detail with a urologists to determine whether active treatment is necessary, or whether surveillance may be an option.
Surgery |
| |
|
| When is surgery the best treatment for prostate cancer? |
|
|
In general, prostate cancer surgery is best performed in patients with clinical stage T1 or T2 prostate cancer (confined to the prostate gland) and in selected men with clinical stage T3 disease. While there are no absolute cut-offs, men with a PSA level less than 20 ng/mL and a Gleason score of less than eight have a higher likelihood of cure. In certain circumstances, patients with more serious parameters are offered surgery. Prostate cancer surgery is usually restricted to men who are healthy enough to tolerate a major operation and have a 10-year or more life expectancy. Life expectancy is assessed by both patient age and health.
What types of surgeries are available to treat localized prostate cancer?
• Open Radical Prostatectomy
• Perineal Radical Prostatectomy
• Retropubic Radical Prostatectomy
• Minimally Invasive
• Robotic Assisted Laparoscopic Radical Prostatectomy
• Laparoscopic Radical Prostatectomy |
| |
|
| What is Radical Prostatectomy? |
|
|
A radical prostatectomy is the surgical removal of the entire prostate, the seminal vesicles, the nearest portions of the vas deferens, the tissue immediately surrounding them, and some of their associated pelvic lymph nodes. Because prostate cancer may be scattered throughout the prostate gland in an unpredictable way, the entire prostate must be removed to ensure that cancer cells are not left behind to continue to grow.
The pelvic lymph nodes are small oval or round bodies located along blood vessels that filter lymphatic fluid. For prostate cancer, the cascade of cancer spread is usually first to the soft tissues surrounding the prostate capsule, then to the seminal vesicles, then to the lymph nodes, and then to the bones and other organs of the body). There are many other lymph nodes, so the body will not be compromised by the removal of these few lymph nodes.
The surgery requires anesthetic, and the patient will be in the hospital for one to three days and then sent home with a urinary drainage tube (a catheter) in his bladder. The tube will be removed after one to two weeks. |
| |
|
| What is the Retropubic Radical Prostatectomy? |
|
|
In the perineal approach, the prostate is removed through an incision in the perineum behind the scrotum. By avoiding the complex pelvic veins, which can cause significant bleeding during the retropubic, laparoscopic, or robotic approaches, bleeding is uncommon with the perineal approach.
What are the advantages and disadvantages of the perineal radical prostatectomy approach?
Advantages to the perineal procedure include:
• precise urethra-vesicle anastomosis (re-attaching the urethra to the bladder),
• a smaller incision,
• generally less pain
The main disadvantages to the perineal approach include:
• higher incidence of rectal injury,
• difficulty of preserving the neurovascular bundles (muscles that control erections)
• a need for a separate incision for pelvic lymphadenectomy (surgical removal of one or more groups of lymph nodes)
However, most patients do not need a lymphadenectomy due to their very low risk of lymph node involvement. Typically, the perineal approach is preferred in obese individuals or those with prior lower abdominal surgery. In general, few urologic surgeons have significant experience with this approach |
| |
|
| What are the advantages and disadvantages of both surgical procedures? |
|
|
The main benefit of a radical prostatectomy is that once the prostate is removed so is all of the cancer if it had not spread to other parts of the body.
A radical prostatectomy has an advantage of providing accurate local staging. This means a doctor can determine the true extent of the disease including whether it has moved beyond the confines of the prostate as well as the potential for assessment of pelvic lymph nodes to determine if the cancer has spread to them.
For patients with prostate cancer pathologically confined to the prostate, the chance of cure with surgery alone at 10 years (undetectable PSA) is more than 90 percent. For men who have cancer progression (cancer beyond the capsule of the prostate gland) while surgery is a good option they may benefit from additional therapy. A recently published randomized trial showed men with metastasized prostate cancer improved their cure rate with the addition of radiation.
Radical prostatectomy has been compared to watchful waiting and has been shown to improve a man’s chance of survival. In a study, conducted in Scandinavia, men with clinically localized prostate cancer were randomly assigned to radical prostatectomy or to watchful waiting groups. In the study, men treated with surgery had a significant improvement in survival. Nonetheless, three additional observations were: (1) most men whose tumors were watched did not die from prostate cancer, (2) some men who underwent surgery did die from prostate cancer, and (3) about 20 operations were needed to save one life from prostate cancer.
The main disadvantage of the radical prostatectomy procedures are the complications that could arise from the operation itself; some occurring early and some late. Bleeding can occur in any major operation. Some surgeons recommend that the patient donate his own blood before surgery or receive a blood-stimulating hormone (Epogen, EPO) to boost his blood count and reduce the risk of needing a blood transfusion from an anonymous donor. Deep venous thrombosis (blood clots in the leg veins or pelvic veins) and pulmonary embolism (blood clot that goes to the lung) occur in approximately 1-2% of patients after radical prostatectomy.
Erectile dysfunction and urinary incontinence are the problems reported most often from this procedure. The chance of having erectile dysfunction depends on a main’s age and health, his sexual function before treatment, the stage of the cancer, and the ability to save the nerves that control erection during surgery. Younger men (those under the age of 60) are less likely to have problems with their erections than are older men. Even if erectile dysfunction does occur after surgery, erections may return to normal over time. There are also medications and devices to treat the problem that may be helpful. You may wish to speak to your doctor about various treatments.
Short-term incontinence after radical prostatectomy is also a common side effect. Many men will require a protective pad for several weeks to months after surgery. Fortunately, most men will ultimately recover urinary control. Long-term (after 1 year) incontinence is rare with occurrence in less than 5-10 percent of all surgical cases. However, when it does occur, there are procedures that can solve the problem.
Other problems reported by patients include irritation of the bladder, gastrointestinal symptoms, bladder infection, blockage of the urine flow from the bladder and leaking of urine. Sometimes scarring can occur and scar tissue may form in the bladder and urinary tract, changing the flow of urine. If a man’s urine flow is blocked, more surgery may be required. |
| |
|
| What is the Robotic Assisted Laparoscopic Radical Prostatectomy? |
|
|
With recent advances in minimally invasive surgery and computer technology, the prostate gland can now be removed through several one- to two-inch incisions in the patient's abdomen. Robotic prostatectomy utilizes a surgical robotic system (a device that holds surgical instruments and a laparoscopic camera to remove the prostate gland through laparoscopic access.
The primary advantage to this approach is less blood loss although transfusion rates do not appear to be different from open prostatectomy approaches when performed by an experienced surgeon. Initially, shorter hospital stays were seen with this approach but with improvements in open surgery, one-day hospital stays are the norm for the open surgery at many institutions. |
| |
|
| What is the Laparoscopic Radical Prostatectomy? |
|
|
Laparoscopic prostatectomy is a type of 'minimally-invasive' surgery that uses six 1-inch incisions, with one being slightly larger in order to extract the prostate gland from the abdomen. Through the small incisions, surgical instruments, including a camera, are inserted. The camera allows the surgeon to view inside the abdomen and perform the surgery while using laparoscopic instruments instead of the robotic arms. This technique is more technically challenging for the surgeon but, in experienced hands, has results that are similar to the robotic technique. |
| |
|
| What are the advantages and disadvantages of the minimally invasive surgeries? |
|
|
It is claimed that laparoscopic and robotic surgery is less invasive and that with the smaller incisions, the patient may experience less pain and scarring and a faster recovery than with the radical prostatectomy approaches. However, in another sense, it is more invasive, because the laparoscopic and robotic operations are performed within the peritoneal cavity (inside the belly where the intestines, major blood vessels, and ureters are located). Thus, the complications of laparoscopic prostatectomy are often more serious, and studies have shown that the recovery time is the same for the laparoscopic and open surgical approaches. Laparoscopic and robotic prostatectomy is difficult to learn and requires special training to perform the operation successfully; meaning to have a better outcome you need a highly qualified and trained surgeon.
Long-term outcomes of laparoscopic or robotic prostatectomy are not yet available as they are for open prostatectomy, and it is uncertain whether the simultaneous preservation of potency and continence with complete removal of the cancer can be achieved as consistently as with the classic radical retropubic prostatectomy. Recent studies have suggested that cancer recurrences rates are higher, urinary continence rates are lower, and patient satisfaction are significantly less with laparoscopic and robotic surgery. |
| |
|
| What can be expected after surgical treatment? |
|
|
After the prostate has been removed and the urinary tract and the bladder have been reconstructed; a urinary catheter is passed through the urethra into the bladder to drain the urine while the new connection between the bladder and urethra (called the "anastomosis") heals. The catheter will remain in place for one to two weeks after the surgery.
One or two suction drains are left beside the bladder, deep in the pelvic cavity, and brought out through the lower abdomen to drain any fluid that might accumulate in the surgical wound. They help to decrease the risk of infection and pressure from fluid in the operated area. The drains are usually removed before you are discharged from the hospital.
Most people do not pass flatus (intestinal gas) for one to two days and do not have a bowel movement until the third day after surgery, depending on how much narcotic pain medication they have had.
The goal during the first few days after your operation will be to prevent the breathing and circulation problems that can develop after any surgery. You must walk at least 100 yards three to four times a day to help your breathing and circulation.
The catheter is removed on a return visit to the surgeon's clinic, and exercises to strengthen the urinary control valve (called Kegel exercises) are begun by the patient after the catheter has been removed.
The surgeon reviews the final pathology report of the removed prostate and (if applicable) the lymph nodes. Based on this "final pathology," a follow-up plan is developed. If the pathology report is favorable, the follow-up plan entails regular visits to a physician and a regular PSA test (every 6-12 months). The post-operative PSA level should be in the "undetectable" range (less than 0.1 ng/mL).
If the pathology report shows adverse features (e.g., cancer at the surgical margin or spread of cancer through the capsule of the prostate into the surrounding tissues, seminal vesicles, or lymph nodes) additional therapy may be recommended or at least considered as an option. This may include postoperative radiation therapy and/or hormone treatment beginning 2 to 4 months after surgery.
There is a risk of developing curvature of the penis (Peyronie's disease) due to scarring from repeatedly injecting into the same site or from kinking or buckling of the penis while having intercourse without a sufficiently rigid erection.
The ability to experience climax (orgasm) is not lost after radical prostatectomy, even in the absence of an erection. However, with orgasm, there is very little (if any) ejaculate (usually some lubricating mucus from urethral glands, and sometimes urine, if urinary sphincter functions, has not fully recovered).
Because the prostate and seminal vesicles have been removed and the vas deferens has been divided, the patient is no longer able to initiate a pregnancy through sexual intercourse (but fertility is still possible through artificial insemination techniques). |
| |
|
| Why is erectile dysfunction a side effect of the majority of treatments for prostate cancer? |
|
|
Erection of the penis occurs because of the stimulation through the nerves that run adjacent to the prostate and send signals to dilate the blood vessels in the penis, allowing it to fill with blood and become rigid. The two nerve bundles responsible for erection run only a few millimeters away from the area where prostate cancer most commonly arises. Although preserving these nerves at the time of surgery is usually possible, it is not always wise. The less tissue removed around the prostate, the greater the chance cancer cells will remain. Since the primary goal of all prostate surgeries is to remove the cancer, often one or both of these nerves are completely or partially destroyed. Unless both nerves are sacrificed, the chance of recovering erectile function exists, but recovery may be slow. The average time until recovery of erections sufficient for intercourse is 4 to 24 months, but in some men, it takes longer.
Of course, the operation does not make erections better than they were before surgery, even if both nerves are spared. Even with full recovery, most men find the erections are less rigid and durable than before surgery. Younger men recover sooner, and those with stronger erections before the operation have a better chance of recovery than if the erections were weak preoperatively.
Erectile rehabilitation programs are usually encouraged beginning after surgery. There also many post-operative treatments that can help erectile dysfunction such as:
o pills,
o vacuum pumps,
o urethral suppositories,
o penile injections;
o surgical implantation of a penile prosthesis
However, these treatments do have some side effects. In general, pills are not very effective until spontaneous erections begin to return. Vacuum pumps draw blood from the veins rather than the arteries into the penis and therefore provide less oxygenation to the tissues. Intraurethral suppositories may be inefficiently absorbed through the urethra and sometimes cause urethral burning. Intracavenosal injections of vasodilators usually provide immediate rigid erections with well-oxygenated arterial blood, but if too large a dose is given, it might induce an erection that will not go away (priapism) and require a visit to the emergency room for treatment.
|
| |
|
| When can I resume normal activity after the surgery? |
|
|
The time varies, but usually it is between three to six weeks. |
| |
|
| Will I know if I am cured after surgery? |
|
|
Not with absolute certainty. The likelihood of cure varies, depending on the severity of the cancer removed. In general, one must have PSA test values of less than 0.1 ng/ml for 10 years before cure is virtually certain.
|
| |
|
| I worry about potency but I am most afraid of incontinence. What are the odds? |
|
|
That depends mostly on the surgeon and his/her experience. Nevertheless, age and your current level of continence and potency are also key factors. Usually, incontinence is temporary and does not last long although it can persist for as much as six to twelve months. With more experienced surgeons, the risk of permanent incontinence is rare after prostate cancer surgery.
|
| |
|
| What is Radiotherapy? |
|
|
Radiotherapy for prostate cancer can be divided into two types:
external beam radiation therapy
brachytherapy
External beam radiotherapy is when a small amount of radiation is delivered in daily increments to the prostate, over a course of 7 to 8 weeks. Currently, three-dimensional conformal radiotherapy (3DCRT) or intensity-modulated radiotherapy (IMRT) is used to deliver high-dose radiation to the prostate while minimizing the damage to the surrounding normal structures such as the bladder and rectum.
Prostate brachytherapy is a radiation technique in which radioactive seeds are implanted directly into the prostate. The seeds are delivered through the perineal skin into the prostate using needles that are inserted into the prostate using ultrasound guidance. Both low-dose rate (but high-dose) permanent prostate seeds and high dose rate (HDR) temporary implants can be used to treat the gland successfully. Prostate brachytherapy is typically performed in an outpatient setting under either general or regional anesthesia. The procedure is usually well tolerated with a low risk of surgical complications.
|
| |
|
| What are the advantages and disadvantages of radiotherapy? |
|
|
The relative effectiveness of external beam radiation and brachytherapy appear to be similar for early stage prostate cancer. Some patients are offered the combination therapy in which both external beam radiation and brachytherapy are utilized. For men with locally advanced cancer and/or aggressive (e.g., Gleason score of 7 or more) cancer, androgen deprivation is also added to improve cancer control.
For more information on advanced prostate cancer and its treatments please refer to our advanced prostate cancer information: Advanced Prostate Cancer
The main side effects of radiotherapy include bladder and rectal toxicities, which can result in urinary and bowel dysfunction. Erectile dysfunction is also common and appears to increase with time after treatment. The long-term effects of radiation to normal tissues remain unknown although men who elect this treatment do have a higher risk of recurrence.
|
| |
|
| What is Cryotherapy? |
|
|
Cryoablation for prostate cancer involves the controlled freezing of the prostate gland in order to destroy cancerous cells. Cryotherapy is administered in a manner similar to that of prostate brachytherapy. Special needles called "cryoprobes" are placed into the prostate transperineally under the guidance of transrectal ultrasound. Argon gas is then used to create an "iceball" which results in cell death within the predefined area. Real-time ultrasound monitoring of cryoablation combined with the use of thermocouples reduces the risk of injury to the surrounding normal tissues.
Cryoablation however is not cancer specific, and the treatment will affect all of the cells that are in the targeted area. The damage caused by freezing occurs at several levels: molecular, cellular and whole tissue structure. In particular, cryotherapy kills targeted tissue by three modes of cell death: (1) cell trauma (secondary to intracellular ice formation), (2) necrosis (immediate cell death and delayed death secondary to vascular stasis preventing oxygen to tissues), and (3) apoptosis (programmed cell death). In addition, there may be an immunological effect when the cancerous cells are killed. Important factors influencing freezing injury are the rate of temperature reduction after the initiation of freezing, the end-temperature reached and performing two freeze-thaw cycles.
The cells are not the only structures damaged during freezing. During cryoablation of the prostate, the surrounding connective tissue (stroma) and the smallest blood vessels (capillaries) are damaged and subsequently have an inadequate blood supply that is also believed to eradicate cancer.
|
| |
|
| Who are the most suitable candidates for cryoablation of the prostate? |
|
|
Suitable candidates for this procedure are patients who have organ-confined prostate cancer or those who have minimal spreading beyond the prostate (clinical stage up to T3a). This includes patients undergoing prostate cancer treatment for the first time and those with recurrent cancer following radiation treatment (external beam or brachytherapy). One benefit of cryotherapy is that it is equally effective when ablating (removing) cancers of any Gleason grade. Due to the ability to use smaller needles, it may be possible to eradicate an area of the prostate that contains the cancer rather than treating the entire prostate gland. This new form of focal cryotherapy is emerging and may change the way that prostate cancer is treated in the future.
|
| |
|
| How is the procedure performed? |
|
|
Under anesthesia (either general or spinal), an ultrasound probe is guided into the rectum. The prostate is imaged and its dimensions measured. An aiming grid software program is then activated and images of the prostate are projected on a screen. Under continuous monitoring with ultrasound imaging, cryoablation probes or needles are placed at predetermined sites within the prostate. Each of the commercially available cryosurgical systems has a different type of probe and placement strategy, but all aim to freeze the prostate, tumor(s) and surrounding tissue—except the urethral area. A urethral warming catheter is used to keep the urethra warm through the procedure. It is kept active for about 20 minutes after the final thaw cycle to prevent the urethra from freezing. In addition to the freezing probes, small temperature probes are also placed in and around the gland to monitor the temperature of the rectal wall as well as other sites such as the urinary sphincter. This has led to a dramatic reduction in side effects such as urinary incontinence and rectal fistula formation.
Prior to the freezing process, cystoscopy (direct visual inspection of the bladder using a small telescope) is performed to ensure cryoprobes have not inadvertently pierced the urethra, if so the probes are simply repositioned. A commercially available urethral warming catheter is placed at this time thereby protecting the urethra from freezing. This is important, as it minimizes the risk of urethral damage, obstruction and urinary incontinence.
Freezing starts at the anterior part of the prostate by activating the anterior probes, followed by the middle and finally the posterior probes. This sequence allows continuous monitoring (by visualizing the freezing process through the transrectal ultrasound) and sculpting of the ice balls. The physician knows when to stop freezing using both the ultrasound image as a guide as well as monitoring the temperature probes. Two freezing cycles are usually done. Between them, the prostate is allowed to thaw passively or actively either using helium or argon gas. If the prostate is longer than the active portion of the cryotherapy probe, an apical pullback maneuver is usually done to freeze the apex of the prostate. Double freezing is performed again. Following the final thaw, either a urethral Foley catheter or a suprapubic catheter (a small tube that is pierced into the bladder through a small opening in the lower abdomen) is inserted and secured in place. The physician will typically remove this catheter several days after cryotherapy when the patient is able to urinate. For most cases, the procedure can be performed under 2 hours.
|
| |
|
| What is the follow-up procedure after this treatment? |
|
|
A PSA test is usually done at three months. In addition, a prostatic biopsy may be recommended some time after the procedure to assess for prostate destruction and absence of viable cancer cells especially if PSA level continues to climb. Once the PSA level has stabilized, the PSA may be checked every 6 months or annually. Of course, if the PSA level is in a state of flux, it will likely be monitored more closely by your physician
|
| |
|
| What are the side effects from the cryotherapy? |
|
|
Cryoablation of the prostate is currently an outpatient procedure. The patient is usually discharged from the recovery room with either a urethral catheter or a suprapubic tube in place for drainage.
Prostate cryoablation will cause the prostate to swell in the short term. Once the swelling has resolved, typically several days to a few weeks after the procedure, the urinary catheter may be removed. The patient has to demonstrate that he is capable of urinating on his own, or the catheter may need to be reinserted again until the prostate swelling has had sufficient time to resolve. Most patients are able to urinate in about 5 to 15 days but some may require longer recovery periods. Oral antibiotics and other medications that help with urination or reduce catheter irritation may be given after treatment, depending on physician preference.
New technological advances have resulted in a significant reduction of the rate of complications. An improved FDA-approved urethral warming device has minimized urethral complications. Better spacing of the probes now contributes to the effectiveness and safety of the procedure. Improved monitoring of the freezing with real-time transrectal ultrasound and temperature thermisters has given the treating physician control over the size and shape of the ice balls formed.
However, some risks still exist. Perhaps one of the most critical is the risk of urinary rectal fistula, which creates a channel between the urethra and the rectum and may cause diarrhea due to urine in the rectum and possibly severe infection due to bacteria in the bladder. However, this has largely been a complication using older technology and is rarely seen today. In fact, a recent study that included over 1000 patients in the COLD registry (CryoOn Line Database) revealed that the rectal fistula rate was only 0.4%. There is also a high incidence of erectile dysfunction when freezing the entire prostate. However, several physicians have developed methods to better preserve erections in those patients who are candidates for a nerve-preserving procedure. Other complications, although uncommon given technological advances, include urinary incontinence, urinary retention requiring transurethral resection of the prostate (TURP) and inflammation of the testicle. Almost all patients have a temporary need for a catheter to empty the bladder for several days after the procedure. Permanent, severe incontinence is rare (approximately 1 percent) and other rare complications include prostatic abscess and permanent penile numbness.
Other less common side effects that the patient may experience are scrotal swelling, numbness at the tip of the penis, passage of flecks of tissue, pain or burning sensation during urination and increased urinary frequency and/or urgency. Most men are pleasantly surprised that there is little to no pain after treatment, and recovery to normal health typically occurs within that first week. Of course, the most common symptoms that a man may experience are those related to having a catheter: urinary urgency and a minor amount of blood in the urine.
|
| |
|
| What are the advantages and disadvantages of cryoablation of the prostate? |
|
|
Cryoablation therapy offers:
• a minimally invasive, outpatient procedure
• favorable success rate
• low toxicity profile (complication rate)
• high quality of life
• a short recuperation period
• No blood transfusions
• Minimal anesthesia
• can be effectively used for high grade cancers
• the ice can be extended beyond the confines of the prostate to achieve an adequate margin
• prostate cancer resistant to radiation, hormones or chemotherapy may be vulnerable to the physical trauma of ice
• may be used as primary treatment or in those who have failed radiation treatment
• procedure can be repeated if the first cryoablation has not completed killed the cancer
• radiation therapy, radical prostatectomy, or hormonal therapy are still options if the procedure fails
• less than half the cost of the traditional treatment
The disadvantages are:
• extensive experience and training by the surgeon are required
• long term outcomes using current technology are still needed
Is cryoablation therapy ever used after other prostate cancer treatments have been tried?
Yes, an important use of cryoablation therapy is for patients who fail or develop recurrence after radiation therapy (external beam or brachytherapy).
|
| |
|
|
| |
|
|
 |
Endoscopic removal of urinary stones: PCNL, URS, RIRS, CLT |
|
 |
LITHOTRIPSY (ESWL) |
|
 |
LASERS for stones and Prostate |
|
 |
Monopolar and bipolar TURP |
|
 |
HOLEP |
|
 |
Urodynamics and uroflowmetry |
|
 |
Laparoscopic urology surgeries |
|
 |
Paediatric urology surgeries |
|
 |
Urinary incontinence surgeries |
|
 |
Surgeries for genitourinary cancers |
|
 |
Reconstructive urology |
|
 |
Microsurgeries for infertility and impotence |
|
| |
|
| |
 |
| |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
|